Clinical Relevance of Connective Tissues: Fibroblasts & Myofibroblasts
Connective tissue is one of the four major classes of tissue (the others being epithelial, muscle and nerve tissue). As its name, it connects or binds cells and tissues, and regarded as the glue that holds the body parts together. Connective tissue maintains and supports the form of the body and its organs. Connective tissue has three major components: cells, fibres and extracellular matrix (ECM). The cells provide the metabolic properties of the tissue, the fibres contribute to the mechanical properties, and the ECM give the plasticity and malleability of the tissue. The most common types of cell are fibroblasts. Fibroblasts synthesize the extracellular matrix and collagen, the structural framework (stroma) for tissues, and have a critical role in wound healing.
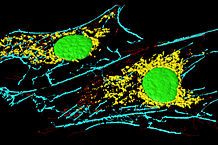
“Fibroblastid (BPAE)” by Heiti Paves. Licensed under CC BY-SA 3.0 via Commons
Fibroblasts make collagens, glycosaminoglycans, reticular and elastic fibres, glycoproteins found in the extracellular matrix, and cytokine. Fibroblasts can differentiate into different cells, such as chondroblasts, which are responsible for making cartilage, and osteoblasts, which produce bone. Fibroblasts have a role in the remodelling of the matrix through degradation and synthesis of new fibres and proteins. Damage in the tissues stimulates fibrocytes and induces the proliferation of fibroblasts.
Mechanical load influences the activity of the fibroblasts and the deposition of collagen fibres. After a sprain or trauma of the musculoskeletal system, new collagen fibres will be produced. However, if the patient is immobilized, the collagen fibres will have an irregular disposition, which can cause restricted movement and delay recovery time. Early movement can remedy the formation of collagen fibres along the functional lines of force (Stecco, 2014).
In an experiment, Loghmani and Warden (2009) injured bilaterally the medial collateral ligaments (MCL) of 51 rodents and used instrument-assisted, cross-fibre massage was applied unilaterally on the injured contralateral ligaments of 31 rodents one week following injury (contralateral injured MCL served as non-treated controls). They cross-friction massaged the injured area three sessions per week for one minute per session. They showed that the treated ligaments were 43% stronger, 40% stiffer and could absorb 57% more energy compared to the contralateral, injured, non-treated ligaments four weeks post-injury. Histological and scanning electron microscopy assessment showed that the treated ligaments have improved collagen fibre bundle formation and orientation within the scar region compared to the non-treated ligaments.
In a subsequent study, Loghmani and Warden (2013) used cross-friction massage on injured MCLs and found that there was not only a temporary increase in vasodilation within the ligament but an alteration of microvascular morphology in the vicinity of the healing knee ligaments, including a larger proportion of blood vessels in the diameter range of arterioles. These changes persisted for one week after final intervention.
Tendons that undergo high rates of stretching may be more susceptible to inflammation and eventual degeneration due to the stretching of fibroblasts. Cyclic stretching of fibroblasts, and especially increasing the frequency of the stretching, increases the production of proinflammatory cyclooxygenase enzyme and prostaglandin-E2. Overstimulating the fibroblasts may be responsible for repetitive-stress injuries (Stecco, 2014).
Several pain syndromes, such as runners’ knee, tennis or golfer’s elbow, frozen shoulder, and plantar fasciitis are related to the stiffening of connective tissue. Histological examinations show accumulations of fibroblasts or contractile myofibroblasts. Whether these cells are contributors to the pathogenesis of connective tissue induced contractures is yet to be known (Klinger et al., 2014). A study by Gibson et al. (2009) suggested that fascia rather than muscle tissue is important in delayed onset muscle soreness (DOMS) associated sensitisation.
Kaux et al. (2013) demonstrated that eccentric exercises may be more beneficial than concentric exercises in the rehabilitation of muscles and tendons. It is believed that the effect of the load pattern of eccentric exercise creates greater stimulation of fibroblasts, which increases collagen synthesis and thus stimulates the healing of the injured tissue. Stretching also causes an increase in tendon fibroblast alignment.
However eccentric exercises are painful to execute and in athletes, eccentric exercises can even increase the risk of tendon pain. Rio et al. (2015) proposed the use of isometric contraction. They investigated heavy isometric quadriceps muscle contractions for their ability to induce immediate analgesia in 6 athletes with patellar tendon pain. They concluded that a single resistance training bout of isometric contractions reduced tendon pain immediately for at least 45 min postintervention. Rio concluded that tendons seem to love heavy isometric load and it reduced tendon pain immediately (Rio, 2015).
Myofibroblasts
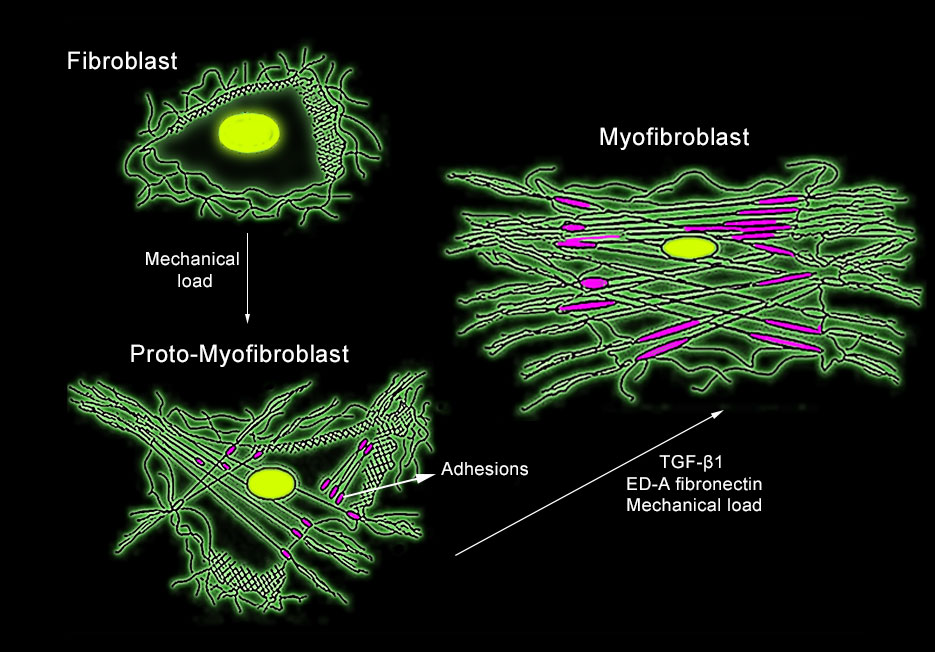
Development of myofibroblasts (Redrawn from from Tomasek et al. 2002, Nature Reviews Molecular Cell Biology)
Myofibroblast, a specialised type of fibroblast, can be found in tendons, fascia (fascia lata, plantar fascia & lumbar fascia) and scars. Robert Schleip and his colleagues at the Fascia lab, found that these cells, invested in fascia, can contract like a smooth-muscle: slow and long-lasting (minutes to hours). The contractility of the cells depends on mechano- stimulation and influence of specific cytokines (groups of proteins, peptides or glycoproteins that are secreted by specific cells of the immune system). The contraction forces may be strong enough to influence musculoskeletal dynamics. Thus, imbalance of this regulatory mechanism can result in altered myofascial tonus, or diminished neuromuscular coordination, which are key contributors to several musculoskeletal pathologies and pain syndromes.
Tom Myers wrote that the matter in which the myofibroblasts contracts and tenses the matrix of ECM will lead us to the wonderful world of cellular tensegrity. Regular fibroblasts are not able to create tension on the ECM, however under mechanical stress, certain fibroblasts can differentiate into proto-myofibroblasts which build more actin fibres and connect them to adhesion sites. Further mechanical and chemical stimulation can result in fully differentiated myofibroblasts. The implications concern the body’s ability to adjust to “pre stress”, a middle ground between contraction of muscles and myofibroblasts contraction. This pre-stress can prepare the body for greater loads or facilitate the transfer of loads from one fascia to the other (Myers, 2013).
Following an injury or inflammation, immune cells invade damaged skeletal muscle areas to phagocytose tissue debris and to release signalling molecules to attract further immune cells. Cytokines and growth factors released from immune cells during the inflammatory response stimulate the migration of fibroblasts into damaged tissue regions and trigger the switch to become myofibroblasts. Myofibroblasts, as indicated above, express a smooth-muscle-type actin–myosin complex that closes wounds and speeds up their repair by contracting the edges of the wound. Upon resolution of the injury, these myofibroblasts undergo apoptosis (programmed cell death). If the wounds fail to resolve, myofibroblasts persist and produce excessive ECM components such as collagen and fibronectin. This cause fibrosis, the development of scar tissues.
A better understanding of the cellular dynamics involved in fibrosis can help direct therapeutic treatments (Klinger et al., 2014). Myofascial therapies can assist in the process of improving matrix remodeling. For example, Bove et al. (2012) demonstrated in a rat model that myofascial massage treatment can lyse, as well as prevent, intra-abdominal postsurgical adhesions.
References
Bove GM. Chapelle SL Visceral mobilization can lyse and prevent peritoneal adhesions in a rat model. J Bodyw Mov Ther. 2012;16:76–82.
Gibson W et al. Increased pain from muscle fascia following eccentric exercise: animal and human findings. Exp Brain Res. 2009;194:299–308
Kaux, J.F., Drion, P., Libertiaux, V., et al., 2013. Eccentric training improves tendon biomechanical properties: a rat model. J. Orthop. Res. 31 (1), 119–124.
Klingler, W., Velders, M., Hoppe, K., Pedro, M. and Schleip, R., 2014. Clinical relevance of fascial tissue and dysfunctions. Current pain and headache reports, 18(8), pp.1-7.
Loghmani, M.T., Warden, S.J., 2009. Instrument-assisted cross-fiber massage accelerates knee ligament healing. J. Orthop. Sports Phys. Ther. 39 (7), 506–514.
Loghmani, M.T., Warden, S.J., 2013. Instrument-assisted cross fiber massage increases tissue perfusion and alters microvascular morphology in the vicinity of healing knee ligaments. Complement. Altern. Med. 28 (13), 240.
Myers, T.W., 20139. Anatomy trains: Myofascial meridians for manual and movement therapists, 3rd Edition. Elsevier Health Sciences.
Rio, E., 2015. Isometrics reduce tendon pain. Body in Mind http://www.bodyinmind.org/isometrics-tendon-pain/
Schleip, R., Naylor, I.L., Ursu, D., et al., 2006. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med. Hypotheses 66 (1), 66–71.
Stecco, C., 2014. Functional atlas of the human fascial system. Elsevier Health Sciences.
.