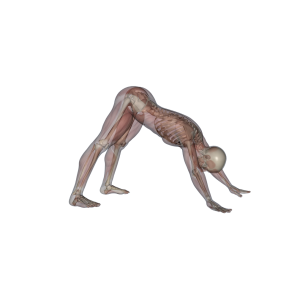Pandiculation: An organic way to maintain myofascial health
 Pandiculation: An organic way to maintain myofascial health by Luiz Fernando Bertolucci, MD
Pandiculation: An organic way to maintain myofascial health by Luiz Fernando Bertolucci, MD
Pandiculation is the involuntary stretching of the soft tissues, which occurs in most animals and is associated with transitions between cyclic biological behaviours, especially the sleep-wake rhythm. Yawning is considered a special case of pandiculation. When, as often happens, yawning occurs simultaneously with pandiculation in other body regions, the combined behaviour is referred to as the stretch-yawning syndrome (SYS).
Although today it is possible to trace the main neural pathways responsible for the expression of the SYS, its intimate biological meanings are still poorly understood. In the First International Congress on Yawning, held in Paris in 2010, different hypotheses were presented about the main possible SYS’s mechanisms and purposes (summarized in the book: The Mystery of Yawning in Physiology and Disease, edited by Dr O. Walusinski), ranging from ethological to neurophysiological perspectives.
This article explores the hypothesis that the SYS has an auto-regulatory role in our locomotor system: to maintain the animal’s ability to express coordinated and integrated movement by regularly restoring and resetting the structural and functional equilibrium of the myofascial system.
The ideas presented here initially arose from clinical observations during the practice of a manual therapy called Muscular Repositioning (MR) (Bertolucci, 2008; Bertolucci and Kozasa, 2010a; Bertolucci, 2010b). These observations were supplemented by a review of the literature on the subject. A possible link between MR and SYS is presented: The neural reflexes characteristically evoked through MR are reminiscent of SYS, which both suggests that MR might stimulate parts of the SYS reaction, and also points to one of MR’s possible mechanisms of action.
Pandiculation: determining and maintaining musculoskeletal structure and function
Pandiculation is an old and almost ubiquitous behaviour that occurs in similar form and circumstances across a wide spectrum of species (Baenninger, 1997). The regularity and vigour of pandiculatory movements suggest that they might be physiologically significant. Walusinki noted that according to Darwin’s concepts, the cost of a behaviour with high metabolic demand is likely to be outweighed by some adaptive benefit (Walusinski, 2006). Indeed, the phylogeny and ontogeny of pandiculation reveal its likely role in the development and maintenance of motor function, in both its structural and neural aspects. Fraser (1989a), in connection with ultrasound foetal studies on sheep, refers to foetal pandiculation as a mechanism that influences functional determination of the moving parts of the musculoskeletal system and contributes to articular development and maintenance. He also identified a bodily care and self-maintenance function of pandiculation, which restores muscle homeostasis in poultry, dogs, cats and horses, among other animals (Fraser, 1989a).
In human, pandiculation is also ontogenetically precocious, starting as early as 12 weeks of gestation (de Vries et al., 1982). It has been associated with the development of motor neural circuitry (Lagercrantz and Ringsted, 2001; Marder and Rehm, 2005; Briscoe and Wilkinson, 2004) and associated musculoskeletal effectors (de Vries et al., 1982; Walusinski et al.,2005). In fact, individual muscles are differentiated only after the establishment of their neural connections (Sadler, 1995), based on their specific motor actions. Such mechanical-structural coupling is not confined to soft tissues; it can be extended to bone (Wolff, 1986), joint surface shapes (Kapandji, 1987) and virtually every tissue under mechanical stress (Moore, 2003; Silver et al., 2003).
In summary of the above, repetitive motion gradually determines shape and composition of moving structures, as well as their associated neural control pathways. The precociousness and stability of pandiculation suggest its contribution to such development.
SYS and arousal: restoration of postural tonus
The SYS has been associated with the maintenance of arousal and attention; i.e., it sets and maintains the central nervous and locomotor systems so that the animal is able to perceive environmental stimuli and respond to them with appropriate motor actions (Baenninger, 1997; Walusinski, 2006; Askenasy, 1989). It has been shown that rats’ stereotypical yawning, including trunk stretching (SYS), can be triggered by stimulation of the paraventricular nucleus (PVN) of the hypothalamus via electrical or chemical means (Sato-Suzuki et al., 1998).
The fact that animals most often awaken immediately following REM sleep (which is characterized by muscle atonia), led Walusinski (2006) to postulate an opposing relationship between REM sleep and SYS. Apparently, SYS restores to the myofascial system the elevated level of tonus required for activity in gravity: because in sleep the myofascia is slack and relaxed, the body segments must be reassembled upon awakening before the organism can move properly in the field of gravity.
SYS: compensatory response to stiffness
The SYS has a similar and stereotyped phenotype along the evolutionary scale, having remained virtually unchanged. Yawning starts with a long and deep inhale, reaches a peak, and concludes with a short exhale. Respiratory, mouth, neck and upper spine muscles engage in co-contraction, simultaneously stiffening the joints and stretching the myofascial tissues (Walusinski, 2006). The few references to body pandiculation in the existing literature describe it as a series of coordinated actions that unfold sequentially, building up soft tissue contractile tension to a peak, at which point the joints of the limbs and trunk are maximally extended or, alternatively, the trunk is arched in flexion (Fraser, 1989b). After the peak, the soft tissue tension level plummets, yielding a sense of pleasure and well-being.
The patterns of full body pandiculation are, in general, similar to the ones used in striding and righting behaviours (Fraser, 1989b) – i.e., they emulate ordinary functional movements – while pandiculation of limited bodily regions seem to be a corrective response to the stiffness induced by temporary positional stress or immobility. Such stiffness is related to the molecular composition and dynamics of the extracellular matrix (ECM). In fact, during physiological turnover metabolism, ECM components are continuously being replaced, depending on the mechanical stresses acting on them; i.e., movement is crucial to the maintenance of the appropriate form and function of the ECM (Kjær et al., 2005, 2006, 2009; Tomiosso et al., 2005). For example, dense tissue is deposited in response to the mechanical need for tensional resistance, while areolar tissue is renewed where gliding is required. Without mechanical input, the ECM would be laid down amorphously and its configuration would not meet physiological requirements.
For the configuration of the ECM to be physiologically appropriate, the mechanical input that stimulates it must first be appropriate. For example, a long striding movement will remain possible only to the extent it is sufficiently expressed, because only such expression will stimulate a supportive ECM configuration (Kjær, 2004; Kjær et al., 2009, Heinemeier et al., 2007). But, most of the time, animals are not expressing their optimal qualities of movement, such as running at maximum speeds and attaining maximum range of movement (ROM) in the joints. What’s more, sleep imposes a regular period of immobilization, to which the ECM turnover will accommodate. This suggests a continual tendency to “tie up” the animal’s entire structure by the creation of abnormal ECM links (adhesions).
Pandiculation, with its specific and vigorous muscle activity, might be a means to compensate for the mechanical signals delivered by rest periods and sub-optimal movements. Fraser mentions, in connection with his studies of pandiculation among various species, that it might be considered a feedback from stiffness, and possibly be triggered by extended periods of immobility in asymmetrical positions. He concludes that if the body tends to stiffen, pandiculation “can serve to restore the limb (and related musculature) to an original (homeostatic) state (Fraser, 1989a, 1989b)”. In fact, SYS has much in common with other homeostatic functions, as discussed below.
Emotional motor system and SYS
Human movement involves the concerted activity of all parts of the motor system. Voluntary movement involves the so-called cortico-spinal tract (connection between cerebral cortex and spinal nerves), which is modulated, by information from lower motor regions (basal ganglia, thalamus, midbrain, cerebellum, spinal cord). Such modulation allows voluntary movements to be smooth, precise and well coordinated (Kandel et al., 2000) and is mainly unconscious and automatic (Jacobs and Horak, 2007; Guyton and Hall, 2006; Takakusaki et al., 2003).
In contrast to voluntary movement, there is a wide range of instinctive motor behaviours that are automatic and can be executed without the intervention by the cortical centres. Instinctive behaviours evolved to guarantee the continuity of the organisms’ lineages, to maintain internal homeostasis, and to insure successful breeding (Dentona et al., 2009). They are mediated primarily by the limbic system and include those life-supporting activities (e.g., feeding, self-defense, sex) collectively named emotional behaviours (Guyton and Hall, 2006; Kandel et al., 2000). In experimental animal models, stimulation of limbic structures, notably the hypothalamus and the amygdala, has elicited various emotional behaviours (Kandel et al., 2000,Guyton and Hall, 2006) – even in the absence of the cerebral cortex. Experimentally, animals without cortical function can feed themselves, express rage and fight, and have sexual intercourse (Magoun and Ranson, 1938; Smith, 1939; Guyton and Hall, 2006), which shows that the limbic system can produce such behaviours in the absence of cortical participation.
In fact, the limbic system sends diffuse and innumerable projections to the medulla. It is a system unto itself, able to produce motor activity independent of the voluntarily driven cortico-spinal system. Holtstege describes it as the emotional motor system because limbically regulated behaviours depend on the emotional state of the animal. The functions governed by the limbic system include different types of involuntary movements associated with olfaction and eating, such as licking, chewing, and swallowing; clonic and rhythmical movements (e.g., locomotion, shivering); sexual function; vocalization, laughing, and crying; and defense reflexes, among others (Guyton and Hall, 2006; Holstege, 1992). Indeed, in laboratory animals various stereotyped behaviors have been reproduced with electrical or chemical stimulation of mesencephalic nuclei, particularly the PVN (paraventricular nucleus) of the hypothalamus. Chewing, licking, lordosis in females, penile erection, and grooming have been observed (Argiolas et al., 2000; De Wied, 1999; Vergoni et al., 1998). The SYS is among these behaviors produced by the limbic system, and its phylogenic longevity suggests an adaptive function. That the SYS is mediated by the PVN suggests that the SYS might serve some general homeostatic function, which functions are carried out autonomously by the emotional motor system.
In human, the workings of the emotional motor system are illustrated by the involuntary movements of patients with voluntary motor pathway lesions. For instance, when hemiplegic patients yawn, they have been observed to raise involuntarily an otherwise plegic arm (Graham, 1982; Töpper et al., 2003; Stewart, 1921) (Omam et al., 1989). Similarly, patients with voluntary facial palsy show facial movements while laughing (Hopf et al., 1992; Töpper et al., 1995; Chernev et al., 2009), and patients incapable of voluntarily opening their mouths do indeed open them while yawning (Askenasy, 1989).
Taken together, the experimental and clinical observations summarized above illustrate that automatic (emotional) motor behaviours can express their patterns independent of the voluntary motor system. Such automatisms may be interfered with, because normal adult human motor behaviour includes the inhibition of instinctual drives (Smith, 1992). In fact, yawning has negative social connotations in most cultures and religious traditions, and the SYS behaviour has been observed to decrease as the person ages (Walusinski et al., 2010). Based on the above, one can speculate that cultural conditioning inhibits the SYS in humans. Given the likely homeostatic function of SYS, any such inhibition might contribute to the high incidence of human musculoskeletal disorders.
Pandiculation versus ordinary stretching: automatic versus volitional motor actions
If we attend to our interoceptive sensations, our experience tells us that pandiculation and SYS exhibit peculiar motor recruitment. If one “yawns” on purpose, one’s internal sensations are quite different from those elicited by a spontaneous yawn. Similarly, the sensations produced by spontaneous pandiculation are different from those that accompany either “volitional pandiculation” or volitional soft tissue stretching. The patterns of volitional stretching are cognitively established and the action purposely performed. They often involve relaxation of the muscles through a diminution of their actions: the subject muscle is elongated passively, as a result of either gravity or the activity of opposing muscles.
By contrast, the patterns of pandiculation are automatic. Through intense and involuntary deep muscle co-contractions, the soft tissues actively elongate themselves against the bony structures as the joints are stiffened. Each movement within the pattern emerges in sequence, apparently from the recruitment of a mosaic of reflexes, the sequence of which can neither be anticipated nor purposely performed. Just as a spontaneous yawn feels quite different from a deliberate imitation of one, spontaneous pandiculation feels quite different from a voluntary pandiculation-like stretch.
Because the voluntary and emotional motor systems have discrete neural pathways, pandiculation’s distinctive internal sensations might be attributable to the motor unit recruitment sequences dedicated to automatic movement patterns. Indeed, the contrast between interoceptive experiences during automatic versus volitional motor actions has been documented (Hommel, 2009). What’s more, operation of the hierarchically higher volitional system can inhibit that of the lower automatic system; this inhibition can disrupt the characteristic spontaneity of the SYS in favor of a cognitively directed stretch. Automatic arm abduction during yawning in hemiplegic shows the non-volitional nature of SYS: the patients did not show any arm movement when they imitated a yawn (Töpper et al., 2003). In other words, voluntarily imitation of the automatic motor pattern (via the cortico-spinal system) will not reproduce immediately the instinctive patterns originated in the limbic system (via the emotional motor system). Moreover, if the motor patterns are discrete, their physiological effects should be discrete, as well.
The importance of stretching to the maintenance of musculoskeletal health is well-known. In humans, each of the myriad of physical fitness regimens that include stretching has its own rationale; and although all muscle groups should be stretched, different regimens address particular problems and are intended to compensate for various patterns of muscle shortness or consequent joint mobility restriction. But how do animals in the wild maintain musculoskeletal health? They perform no voluntary stretching and still maintain their motor capabilities. Might SYS be responsible? If so, and if it were possible to stimulate SYS, might SYS be employed to achieve therapeutic goals?
Various somatic practices encourage the SYS because of its apparent homeostatic effects, e.g., Hanna Somatics, Joyflexing, Eutonia (Hanna, 2004; Johnson, 2002; Vishnivetz, 1995). In Eutonia, the SYS is observed to be evoked by certain attentional states and forms of mechanical stimulation. Similarly, the specific mechanical stimulation of Muscle Repositioning might also stimulate the SYS. (see section on responses induced by MR, below).
Pleasure and health
Ancient biological behaviours associated with the maintenance of homeostasis are directed through interoception – the sensory experience reflective of the physiological condition (Craig, 2003). Sensory experiences of displeasure and pleasure define the affective qualities of stimuli, which influence an animal’s behaviour (Guyton and Hall, 2006; Bozarth, 1994). The positive effects of pleasurable experiences support many life-supporting behaviours: satisfaction of hunger and thirst, sexual intercourse, and vesicle and bowel evacuation are examples of instinctive behaviours that, once accomplished, reward the animal with an experience of pleasure, which biologically reinforces their expression.
Preservation of health (salutogenesis) is intimately linked to the perception of positive affects (Esch and Stefano, 2004). Such positive affect states are closely related to ancient limbic brain regions common among humans and other mammals (Burgdorf and Panksepp, 2006; Vincent, 1994; Cabanac, 1992). The instinctive behaviours, contributing as they do to the maintenance of the internal milieu, can be considered homeostatic drives (Sherwood, 2010), a category within which pandiculation may also be included. Not only has pandiculation been associated with pleasure and well-being (Fraser, 1989a; Sauer and Sauer, 1967; Russel and Fernandez-Doz, 1997; Walusinski, 2006; Steward, 1921), but it also shares with the other homeostatic drives involvement of the PVN nucleus of the hypothalamus. Homeostasis is maintained chiefly by the parasympathetic division of the autonomic nervous system (Recordati and Bellini, 2004), and increased parasympathetic activity has been detected during SYS (Askenasy and Askenasy, 1996). What’s more, the frequency of SYS is correlated with degrees of health or convalescence: Fraser (Fraser, 1989b) notes that pandiculation is absent in animals with some systemic illnesses, but returns as the animal recovers. Similarly, in recovering hemiplegic patients, SYS and synkinesias characteristically re-emerge (Töpper et al., 2003; Hwang et al., 2005) in advance of voluntary limb movements.
However, excessive pandiculation is associated with certain diseases and the use of certain drugs (Askenasy, 1989). This suggests a possible distinction between complete (and successful) and an incomplete (and unsuccessful) pandiculation. Perhaps the former, having fulfilled its purpose, physiologically recurs; while the latter, seeking completion, pathologically repeats. Consider, for example, the palpable frustration that accompanies the interruption of a yawn or sneeze!
The pandiculation connection: yoga and martial arts
The downward dog position, like many yoga asanas, is reminiscent of an animal pandiculation position (Iyengar, 1979). In fact, some say yoga is derived from automatic and spontaneous actions of sages deep in meditation, and that yoga should be practiced spontaneously (Muni, 1994). Eastern martial arts might also have a connection with pandiculation. Qi Gong, for instance, requires the body to be fortified with automatic (involuntary) tonus in the deep postural muscles at the same time the superficial muscles associated with voluntary activity are relaxed. Under these conditions, the body is integrated as a whole and all its parts relate with one another in movement (see http://www.caiwenyu.com.br/09_Fotos_p_ing.htm). These conditions cannot be produced by voluntary motor action, but emerge spontaneously with appropriate states of attention in which mechanosensing is enhanced. A person in such state could take advantage of elastic potential energy stored in the body when performing a blow. This characteristic of Qi Gong suggests a tensegrity-based mode of action with a high pre-stress level. In fact, potentiation of performance has already been shown in prestretched muscles, due to their ability to store potential elastic energy (Bosco et al., 2008; Ettema et al., 1990; Ishikawa et al., 2006). Elements of martial arts training forms are often described in terms suggestive of animal pandiculatory patterns (Johnson, 2002). This invites reflection upon the fact that decorticated cats and dogs do exhibit instinctual behaviours, such as eating, copulating and fighting (Argyle, 1988); i.e., that basic life-supporting behaviours can happen without cortical participation. In fact, fighting appears to be a largely reflexive behaviour, the expression of which is associated with subcortical structures such as the hypothalamus and midbrain periaqueductal gray (PAG) (Ulrich and Azrin, 1962; Shaikh and Siegel, 1994).
Muscle Repositioning: assisting pandiculation?
Like pandiculation, MR’s manual local loading of the myofascial system integrates body parts, apparently by inducing co-contraction of opposing muscle groups (Bertolucci, 2008; Bertolucci and Kozasa2010a, Bertolucci, 2010b), at the same time as it evokes a measurable rise in tonic muscle activity indicative of an overall increase in load. The client’s subjective experience is similar to that evoked by pandiculation, which suggests a common element among pandiculation, yoga and martial arts and MR.
In pandiculation, muscle activation begins locally and spreads to neighbouring areas until it reaches a peak of distribution and intensity; i.e., joints progressively stiffen through a chain of reflexes, in which neighboring segments are sequentially engaged to form an ever-larger block that eventually encompasses the entire body. Following the peak, the tissues release. MR induces a similar progressive engagement of body segments. The inclusion of each segment increases the overall tension within the block until, following the peak, the practitioner feels an abrupt soft tissue release. The progressive segmental engagement is paralleled by an increasing involuntary tonic muscle activity observable both by palpation and by electromyography (Bertolucci, 2008; Bertolucci and Kozasa, 2010a; Bertolucci, 2010b). Let us imagine how it might be that MR and pandiculation would evoke similar muscle activity.
The author hypothesizes that the manual forces applied during MR maneuvers mimic internal forces, and therefore induce mechanoreceptor afferents, similar to those characteristic of pandiculation. In the clinical setting, recipients of MR treatments have exhibited spontaneous pandiculation-like movements (see videos at http://musclerepositioning.blogspot.com/), and have described their subjective experiences during MR as similar to their experiences during pandiculation. Some clients also report having resumed the habit of pandiculating in the morning, to which they attribute a greater sense of bodily well-being along with relief from musculoskeletal symptoms. These observations support the hypothesis of a similarity between MR and pandiculation. Perhaps MR is a blend of myofascial release and “assisted pandiculation”, with the soft tissue release evoked by a combination of the practitioner’s manual input and the internally generated forces of tonic pandiculation-like reactions. This combination of forces might produce a greater effect in the soft tissues than either manual input or pandiculation alone.
Conclusions
The concept of myofascial force transmission (Huijing and Jaspers, 2005) assumes the presence of ECM links among musculoskeletal components, which links unite those components into an integrated system; i.e., the fascia itself is assumed to play an integrative role. Integrated movement both requires and stimulates appropriate matrix connections. However, animals engage in a great deal of non-optimal movement, of which immobilization (e.g., during sleep), trauma and bad postural habits are among the causes. Should normal activities generate both “good” and “bad” mechanical signals to the ECM, the bad signals would need to be countermanded by good ones for the animal to maintain full movement capabilities throughout life. Pandiculation might provide one source of good signals by (i) breaking bad connections while stimulating better ones, and (ii) resetting postural muscle tonus to produce integrated movement, which movement is a further source of good mechanical signals. In short, pandiculation might be a form of neuro-myofascial hygiene. If this be true, might we encourage pandiculation to enhance general health? This would require a reassessment of the cultural stigma against yawning and pandiculation, as well as further investigation of therapeutic approaches, such as Muscle Repositioning, that seem to stimulate it.
About the Author
Luiz Fernando Bertolucci is a physiatrist and co-founder of Núcleo Anthropos, Integrative Medicine group at UNIFESP (São Paulo Federal University). He is also a Certified Advanced Rolfer™; Rolf Institute® of Structural Integration and Associação Brasileira de Rolfing faculty.
This article builds on part of an earlier publication: Bertolucci LF. Pandiculation: nature’s way of maintaining the functional integrity of the myofascial system? J Bodyw Mov Ther 2011 Jul; 15(3):268-80.
References
Argiolas, A., Melis, M.R., Murgia, S., Schio¨th, H.B., 2000. ACTH- and alpha-MSH-induced grooming, stretching, yawning and penile erection in male rats: site of action in the brain and role of melanocortin receptors. Brain Research Bulletin 51 (5), 425-431.
Argyle, M., 1988. Bodily Communication. Routledge, New York.
Askenasy, J.J., 1989. Is yawing an arousal defense reflex? The Journal of Psychology 123 (6), 609-621.
Askenasy, J.J., Askenasy, N., 1996. Inhibition of muscle sympathetic nerve activity during yawning. Clinical Autonomic Research 6, 237-239. doi:10.1007/BF02291140.
Baenninger, R., 1997. On yawning and its functions. Psychonomic Bulletin and Review 4 (2), 198-207.
Bertolini, A., Gessa, G.L., 1981. Behavioral effects of ACTH and MSH peptides. Journal of Endocrinological Investigation 4, 241-251.
Bertolucci, L.F., 2008. Muscle Repositioning: “A new verifiable approach to neuro-myofascial release?”. Journal of Bodywork and Movement Therapies 12, 213-224.
Bertolucci, L.F., 2010b. Muscle Repositioning: combining subjective and objective feedbacks in the teaching and practice of a reflex-based myofascial release technique. International Journal of Therapeutic Massage and Bodywork 3 (1), 26-35.
Bertolucci, L.F., Kozasa, E.H., 2010a. Sustained manual loading of the fascial system can evoke tonic reactions: preliminary results. International Journal of Therapeutic Massage and Bodywork 3 (1), 12-14.
Bosco, C., Komi, P.V., Ito, A., 2008. Prestretch potentiation of human skeletal muscle during ballistic movement. Acta Physiologica Scandinavica 111 (2), 135-140.
Bozarth, M.A., 1994. Pleasure systems in the brain. In: Warburton, D.M. (Ed.), Pleasure: The politics and the reality. John Wiley & Sons, New York, pp. 5-14.
Briscoe, J., Wilkinson, D.G., 2004. Establishing neuronal circuitry: hox genes make the connection. Genes and Development 18(14), 1643-1648.
Burgdorf, J., Panksepp, J., 2006. The neurobiology of positive emotions. Neuroscience Biobehavioral Reviews 30 (2), 173-187.
Cabanac, M., 1992. Pleasure: the common currency. Journal of Theoretical Biology 155 (2), 173-200.
Chernev, I., Petrea, R.E., Reynolds, M.S., Wang, F., 2009. The classical type of Foix-Chavany-Marie syndrome: assessment and treatment of dysphagia. The Internet Journal of Neurology 11 (1).
Chiquet, M., Gelman, L., Lutz, R., Maier, S., 2009. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochimica and Biophysica Acta 1793 (5), 911-920.
Craig, A.D., 2003. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology 13 (4), 500-505.
de Vries, J.I., Visser, G.H., Prechtl, H.F., 1982. The emergence of fetal behavior: I. Qualitative Aspects Early Human Development 7 (4), 301-322.
De Wied, D., 1999. Behavioral pharmacology of neuropeptides related to melanocortins and the neurohypophyseal hormones. European Journal of Pharmacology 375 (1-3), 1e11.
Dentona, D.A., McKinleyc, M.J., Farrell, M., Egan, G.F., 2009. The role of primordial emotions in the evolutionary origin of consciousness. Consciousness and Cognition 18 (2), 500-514.
Esch, T., Stefano, G.B., 2004. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuroendocrinology Letters 4 (25), 235-251.
Fraser, A.F., 1989a. Pandiculation: the comparative phenomenon of systematic stretching. Applied Animal Behaviour Science 23, 263-268.
Fraser, A.F., 1989b. The phenomenon of pandiculation in the kinetic behaviour of the sheep fetus. Applied Animal Behaviour Science 24 (2), 169-182.
Graham, M., 1982. Assoctiated reactions in the hemiplegic arm. Scandinavian Journal of Rehabilitation Medicine 14 (3), 117-120.
Guyton, A.C., Hall, J.E., 2006. Textbook of Medical Physiology. Elsevier, Philadelphia.
Hanna, T., 2004. Somatics: Reawakening the Mind’s Control of Movement, Flexibility, and Health. Da Capo Press, Cambridge.
Holstege, G., 1992. The emotional motor system. European Journal of Morphology 30 (1), 67-79.
Hommel, B., 2009. Action control according to TEC (theory of event coding). Psychological Research 73 (4), 512-526.
Hopf, H.C., Mu¨ller-Forell, W., Hopf, N.J., 1992. Localization of emotional and volitional facial paresis. Neurology 42 (10), 1918-1923.
Huijing, P.A., Jaspers, R.T., 2005. Adaptation of muscle size and myofascial force transmission: a review and some new experimental results. Scandinavian Journal of Medicine and Science in Sports 15 (6), 349-380.
Hwang, I.S., Tung, L.C., Yang, J.F., Chen, Y.C., Yeh, C.Y., Wang, C.H., 2005. Electromyographic analyses of global synkinesis in the paretic upper limb after stroke. Physical Therapy 85(8), 755-765.
Ishikawa, M., Komi, P.V., Finni, T., Kuitunen, S., 2006. Contribution of the tendinous tissue to force enhancement during stretcheshortening cycle exercise depends on the prestretch and concentric phase intensities. Journal of Electromyography and Kinesiology 16 (5), 423-431.
Iyengar, B.K.S., 1979. Light on Yoga. Schocken Books, New York.
Jacobs, J.V., Horak, F.B., 2007. Cortical control of postural responses. Journal of Neural Transmission 114 (10), 1339-1348.
Johnson, R., 2002. Boa forma para preguiçosos: Joyflexing. Editora Pensamento-Cultrix, São Paulo.
Kandel, E.R., Schwartz, J.H., Jessell, T.M., 2000. Principles of Neural Science. McGraw-Hill, New York.
Kapandji, I.A., 1987. Fisiologia articular: Esquemas comentados de mecaˆnica humana. Ed Manole, São Paulo.
Kjær, M., 2004. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiological Reviews 84 (2), 649-698.
Kjær, M., Langberg, H., Heinemeier, K., Bayer, M.L., Hansen, M., Holm, L., Doessing, S., Kongsgaard, M., Krogsgaard, M.R., Magnusson, S.P., 2009. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scandinavian Journal of Medicine and Science in Sports 19 (4), 500-510.
Kjaer, M., Langberg, H., Miller, B.F., Boushel, R., Crameri, R., Koskinen, S., Heinemeier, K., Olesen, J.L., Døssing, S., Hansen, M., Pedersen, S.G., Rennie, M.J., Magnusson, P., 2005. Metabolic activity and collagen turnover in human tendon in response to physical activity. Journal of Musculoskeletal and Neuronal Interactions 5 (1), 41-52.
Kjær, M., Magnusson, P., Krogsgaard, M., Boysen Møller, J., Olesen, J., Heinemeier, K., Hansen, M., Haraldsson, B., Koskinen, S., Esmarck, B., Langberg, H., 2006. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. Journal of Anatomy 208 (4), 445-450.
Lagercrantz, H., Ringstedt, T., 2001. Organization of the neuronal circuits in the central nervous system during development. Acta Paediatrica 90 (7), 707-715.
Lehmann, H.E., 1979. Yawning: a homeostatic reflex and its psychological significance. Bulletin of the Menninger Clinic 43(2), 123-136.
Marder, E., Rehm, K.J., 2005. Development of central pattern generating circuits. Current Opinion in Neurobiology 15 (1),86e93.
Moore, S.W., 2003. Scrambled eggs: mechanical forces as ecological factors in early development. Evolution and Development 5 (1), 61-66.
Muni, S.W., 1994. Awakening the life force: The philosophy and psychology of “spontaneous yoga”. Llewellyn Publications, Minnesota.
Sadler, T.W., 1995. Langman’s Medical Embriology, seventh ed.. Williams & Wilkins, Baltimore.
Sato-Suzuki, I., Kita, I., Oguri, M., Arita, H., 1998. Stereotyped yawning responses induced by electrical and chemical stimulation of paraventricular nucleus of the rat. Journal of Neurophysiology 80 (5), 2765-2775.
Shaikh, M.B., Siegel, A., 1994. Neuroanatomical and neurochemical mechanisms underlying amygdaloid control of defensive rage behavior in the cat. Brazilian Journal of Medical and Biological Research 27 (12), 2759-2779.
Sherwood, L., 2010. Human Physiology: From Cells to Systems, seventh ed.. Brooks/Cole, Belmont CA.
Silver, F.H., Siperko, L.M., Seehra, G.P., 2003. Mechanobiology of force transduction in dermal tissue. Skin Research and Technology 9 (1), 3-23.
Smith, K.U., 1939. The behavior of decorticate guinea pigs. Journal of Comparative Psychology 27 (3), 433-447.
Smith, R., 1992. Inhibition: History and Meaning in the Sciences of Mind and Brain. University of California Press, Berkeley.
Stevens-Tuttle, D., Fox, J., Bouffard, N.A., Henry, S., Wu, J., Langevin, H.M., 2008. Perimuscular fascia remodeling in porcine model relevant to human low back pain. Journal of Bodywork and Movement Therapies 13 (1), 91.
Stewart, J.P., 1921. On muscle-tonus, tonic rigidity, and tonic Fits. British Medical Journal 1 (3137), 217-219.
Takakusaki, K., Ohinata-Sugimoto, J., Saitoh, K., Habaguchi, T., 2003. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Progresss in Brain Research 143, 231-237.
Tomiosso, T.C., Gomes, L., de Campos Vidal, B., Pimentel, E.R., 2005. Extracellular matrix of ostrich articular cartilage. Biocell 29 (1), 47-54.
Töpper, R., Kosinski, C., Mull, M., 1995. Volitional type of facial palsy associated with pontine ischaemia. Journal of Neurology. Neurosurgery and Psychiatry 58 (6), 732-734.
Töpper, R., Mull, M., Nacimiento, W., 2003. Involuntary stretching during yawning in patients with pyramidal tract lesions: further evidence for the existence of an independent emotional motor system. European Journal of Neurology 10 (5), 495-499.
Ulrich, R.E., Azrin, N.H., 1962. Reflexive fighting in response to aversive stimulation1 Journal of Experimental Analysis of Behavior. October 5 (4), 511-520.
Urba-Holmgren, R., Gonzalez, R.M., Holmgren, B., 1977. Is yawning cholinergic response? Nature 267 (5608), 261-262.
Vergoni, A.V., Bertolini, A., Mutulis, F., Wikberg, J.E., Schiöth, H.B., 1998. Differential influence of a selective melanocortin MC4 receptor antagonist (HS014) on melanocortin-induced behavioural effects in rats. European Journal of Pharmacology 362 (2-3), 95-101.
Vincent, J.D., 1994. Biology of pleasure. Presse Médicale 23 (40), 1871-1876.
Vishnivetz, B., 1995. Educação do corpo para o ser. Editora Summus, São Paulo.
Walshe, F.M.R., 1923. On certain tonic or postural reflexes in hemiplegia with special reference to the so called “associated movements”. Brain 46, 1-37.
Walusinski, O., 2006. Yawning: unsuspected avenue for a better understanding of arousal and interoception. Medical Hypotheses 67 (1), 6-14.
Walusinski, O., Kurjak, A., Andonotopo, W., Azumendi, G., 2005. Fetal yawning assessed by 3D and 4D sonography. The Ultrasound Review of Obstetrics and Gynecology 5 (3), 210-217.
Walusinski, O., Meenakshisundaram, R., Thirumalaikolundusubramanian, P., Diwakar, S., Dhanalakshmi, G., 2010. Yawning: Comparative Study of Knowledge and Beliefs, Popular and Medica. In the Mystery of Yawning in Physiology and Disease. Available at. http://www.baillement.com/recherche/beliefs_knowledge.pdf.
Wolff, J., 1986. The Law of Bone Remodelling. Springer-Verlag, Berlin.