Treatments of muscle injuries: Effect of macrophages during skeletal muscle regeneration and hypertrophy
Skeletal muscles are plastic and can adapt to physical activities, but also can be easily injured. Muscle remodels with increasing mechanical loading or contractile activity by increasing its mass and/or myofibre size, referred to as skeletal muscle hypertrophy. Muscle remodelling mainly relies on the adaptation of the myofibers while regeneration relies on muscle stem cells and their neighbourhood, including inflammatory cells, and particularly macrophages.
A review article summarizes the role of macrophages in muscle regeneration and in response to exercise‐induced muscle damage in humans.
Studies on human remodelling or self-repair mechanisms indicate a link between macrophage accumulation and the magnitude of muscle damage. On mild injuries, it is independent of muscle stem cells. However, on severe muscle damage is consistently associated with leukocyte or macrophage infiltration.
In response to resistance training, macrophages are mobilized into the skeletal muscle, even though the molecular regulators involved in muscle hypertrophy are still uncertain.
MANAGEMENT OF THE INFLAMMATORY RESPONSE
Cooling
Cooling therapy, such as topical icing, cold‐water immersion or cryotherapy is commonly used to treat muscle soreness. However, cooling may actually alter skeletal muscle regeneration. Cooling decreases the number of macrophages and delays the shift towards restorative macrophages. Human studies further illustrate that it neither accelerates muscle strength recovery nor reduce muscle soreness after muscle injury.
Heat therapy
Heat therapy following muscle injury is a promising therapeutic approach for promoting skeletal muscle regeneration. Post‐injury heating treatment results in a rapid increase in the number of macrophages, a greater number of muscle stem cells within the damaged muscle and a higher myonuclear accretion together with a faster muscle generating protein expression.
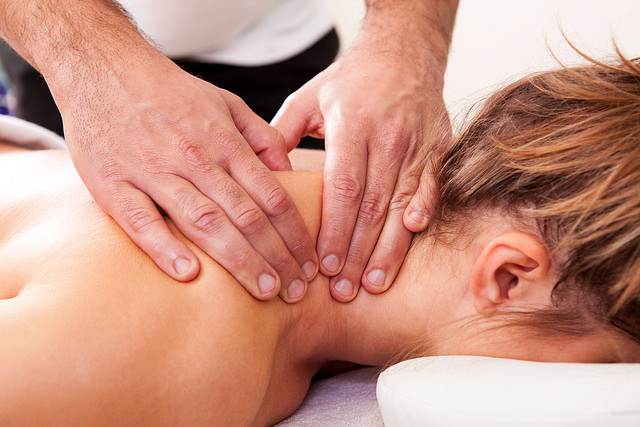
Massage
Skeletal muscle is composed of various cell types than can sense and respond to mechanical loads, a physiological process referred to as mechanotransduction. Research studies on animals and humans have demonstrated massage’s immunomodulatory effects on skeletal muscle homeostasis.
Laboratory experiment shows that the application of controlled cyclic compressive loading in uninjured muscle leads to the expression of genes associated with the immune response. Animal studies on rabbits further demonstrated that four consecutive days of cyclic compression decreases the infiltration of leukocytes or macrophages and facilitates recovery after exercise-induced muscle damage.
The effectiveness of cyclic compression on muscle regeneration depends on the massage timing (i.e., an immediate application is preferred to a delayed intervention) and the magnitude and frequency of mechanical loading.
A study on mice evaluating tissue compression suggests that massage could modulate the inflammatory status of macrophages, reduce the adhesion of pro‐fibrotic macrophages, accelerate immune cell, and remove metabolic waste products through increased fluid transportation.
However, a recent laboratory study showed that cyclic muscle tissue compression‐induced functional improvement is not related to changes in macrophage content and inflammatory status. However the effect is ascribed to early clearance of neutrophils resulting in a reduction of the expression of several cytokines that reduce the proliferation and promote the differentiation of muscle stem cells.
Investigation on human into an exercise-induced injury indicates that massage attenuates the production of inflammatory cytokines and promotes the growth of mitochondria.
Massage‐induced mechanotransduction could be beneficial for increasing muscle mass. Mechanotransduction translates physical forces into biochemical signaling pathways and activates specific transcription factors that promote cell proliferation of muscle stem cells.
Massage could therefore impact macrophages and their microenvironment to promote muscle growth.
In summary
Following muscle injury, cooling or administration of nonsteroidal anti‐inflammatory drugs administration (NSAIDs) can inhibit muscle regeneration and decrease myofiber size. In addition, cooling decreases macrophage number and delays the shift towards anti‐inflammatory or restorative macrophages. Similarly, NSAIDs act upstream to reduce or delay macrophage infiltration by inhibiting cyclooxygenase enzyme activity. As a result of perturbed inflammation, muscle stem cells cannot be properly activated and repair damaged muscle fibres, leading to the accumulation of necrotic muscle fibres in conjunction with collagen/fibrosis deposition.
Contrary, heating or massage have beneficial effects on muscle regeneration. Heating results in rapid increase of macrophages capable of resolving faster inflammation. Massage by applying cyclic movements on the injured muscle reduces the inflammatory infiltrate and favours early clearance of neutrophils.